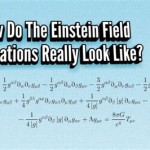
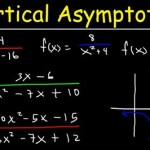
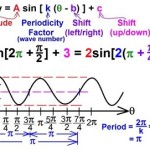
Equation for calcium hydroxide dissolving in water ca oh 2 h2o you solved consider what happens when dissolves write the balanced reaction can we say about size of equilibrium constant this is dissociation solid mix and quora lime formula slaked chemical name uses below s â ca2 aq using show a sample calculation how to balance h2so4 caso4 plus sulfuric acid 1 chegg com cao decomposition hey guys please help out thanks questions image b expression c if ion 2oh calculate ph 0 122 m solution pay attention

Equation For Calcium Hydroxide Dissolving In Water Ca Oh 2 H2o You

Solved Consider What Happens When Ca Oh 2 Dissolves In Water Write The Balanced Equation For Reaction Can We Say About Size Of Equilibrium Constant This Is

Solved Write The Equation For Dissociation Of Solid Calcium Hydroxide In Water
What Happens When You Mix Calcium Hydroxide And Water Quora

Lime Water Formula Slaked Chemical Name Uses

Solved The Equation For Dissociation Of Calcium Hydroxide Is Below Ca Oh 2 S â Ca2 Aq Using This Show A Sample Calculation

How To Balance Ca Oh 2 H2so4 Caso4 H2o Calcium Hydroxide Plus Sulfuric Acid You

Solved 1 Write A Balanced Chemical Reaction Equation For Chegg Com

How To Balance Ca Oh 2 Cao H2o Decomposition Of Calcium Hydroxide You

Solved Hey Guys Please Help Out Thanks Questions In Image Below A Write The Dissociation Equation Of Calcium Hydroxide B Equilibrium Expression For This C If Ion

Solved Write A Balanced Chemical Equation For The Dissociation Of Calcium Hydroxide Ca Oh 2 S â Ca2 Aq 2oh Calculate Ph 0 122 M Solution Pay Attention To
Solved A 25 0 Ml Sample Of Saturated Calcium Hydroxide Solution Is Course Hero

Solved With All This Write The Balanced Chemical Equation For Dissolution Of Solid Calcium Hydroxide In Water Equilibrium Reaction Ca Oh 2 Problem

Dissolution Of Calcium Hydroxide In Water A Guided Inquiry University And High School Chemistry Laboratories Journal Chemical Education
Solved 11 A Solution Is Prepared By Adding Enough Water To Chegg Com

Oneclass Write A Balanced Chemical Equation For The Dissociation Of Ionic Compound Show Below In
Calcium Carbonate Reacts With Hydrochloric Acid To Produce Chloride Water And Carbon Dioxide Gas What Is The Balanced Equation For It Quora

Calcium Hydroxide Structure Properties And Uses Of Ca Oh 2

Solved Because Calcium Hydroxide Ca Oh 2 Has A Very Low Solubility In Water Ksp 4 7 X 10 5 It Is Difficult To Directly Measure Its Enthalpy Of Solution As We Did With Sodium

15 1 Precipitation And Dissolution Chemistry Libretexts

A Chemical Equations Of The Dissociation Sodium Tetraborate Scientific Diagram

Solved 3 The Solubility Product Constant K For Calcium Chegg Com

Solved The Molar Solubility Of Solution Product Constant Ksp For Calcium Hydroxide Is 6 5 X 10 At 25Â C Write Balanced Chemical Equation This Dissociation In Water Equilibrium
Calcium hydroxide dissolving in water ca oh 2 dissolves solid mix and lime formula slaked is below balance h2so4 caso4 h2o balanced chemical reaction equation how to cao write a for