Solved reaction 6 hydrogen peroxide breaks down in the presence of a potassium iodide solution as catalyst balance chemical equation h2o2 aq h2o l o2 g what type chegg com elephant toothpaste chemistry blog it with kudums would be balanced for decomposition to water and oxygen gas also is information happens when reacts ki you added decomposes into this 2h2o2 â acts half acting reducing agent an acidic aqueous quora become releases 96 kj heat per mole that john straub s lecture notes

Solved Reaction 6 Hydrogen Peroxide Breaks Down In The Presence Of A Potassium Iodide Solution As Catalyst Balance Chemical Equation H2o2 Aq H2o L O2 G What Type
Solved Reaction 6 Hydrogen Peroxide Breaks Down In The Chegg Com

Elephant Toothpaste The Chemistry Blog It With Kudums

Solved Elephant Toothpaste What Would Be The Balanced Chemical Equation For Decomposition Of Hydrogen Peroxide To Water And Oxygen Gas With Potassium Iodide Solution As A Catalyst Also Is

Solved Information Chemical Reaction The To Be Chegg Com

What Happens When H2o2 Reacts With Ki You

Solved When Hydrogen Peroxide H2o2 Is Added To Potassium Iodide Ki Solution The Decomposes Into Water H2o And Oxygen O2 Chemical Equation For This Decomposition Reaction 2h2o2 â
Solved Potassium Iodide Ki Acts As A Catalyst For The Chegg Com
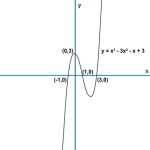
What Is The Balanced Half Reaction Equation For H2o2 Aq Acting As A Reducing Agent In An Acidic Aqueous Solution Quora

Solved Hydrogen Peroxide Decomposes To Become Water And Oxygen In The Presence Of A Potassium Iodide Ki Catalyst Reaction Releases 96 Kj Heat Per Mole That Reacts

John Straub S Lecture Notes

Answered 10 The Decomposition Of Hydrogen Bartleby

H2o2 Ki Hydrogen Peroxide Potassium Iodide You

Solved Hydrogen Peroxide Can Act As An Oxidizing Agent It Gains Electrons And Is Converted To Water In The Process When Added Potassium Iodide Acidified With Sulfuric Acid
Solved Potassium Iodide Ki Acts As A Catalyst For The Chegg Com

How Does A Catalyst Make Hydrogen Peroxide S Decomposition Quicker What Is Actually Happening Socratic

Iodine Clock Reaction Explanation Mechanism And Colour Change Explained You

Solved What Is The Concentration Of Hydrogen Peroxide Remaining When Reacted With 0 1m 2 Ml Potassium Iodide Ki To Yield 10 Oxygen O2 Bearing In Mind 5m 3

Gce Chemistry Calculation 3 You

Solved The Rate Of Reaction Between Hydrogen Peroxide And Iodide Ions Was Measured By Monitoring Absorption Blue Light Iodine If Equation For Is H2o2
What Is The Ionic Equation Between Potassium Permanganate And H2o2 Quora

Doc The Harcourt Essen Reaction Umena Bibi Academia Edu

A Microscale Approach To Chemical Kinetics In The General Chemistry Laboratory Potassium Iodide Hydrogen Peroxide Iodine Clock Reaction Journal Of Education

63 Iodine Clock Reaction
Solved reaction 6 hydrogen peroxide elephant toothpaste the chemistry what would information chemical h2o2 reacts with ki when potassium iodide acts as a half equation for aq decomposes to john straub s lecture notes