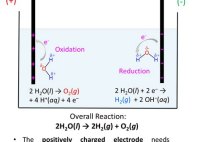
Water Undergoes Electrolysis To Produce Hydrogen And Oxygen Equation
Electrolysis of water chemistry steps solved determine the theoretical and percent yield hydrogen gas if 36 0 g undergoes to produce oxygen 3 80 is collected how many grams will be produced from 5 00g reacting with excess h2 o2 h2o in during are liberated at anode cathode respectively ecomposes into by balanced equation 6 when moles arel… Read More »