Kinetics derive expression for rate constant of first order reaction answer mp board class 12 chemistry question collection what is integrated law gas equation a sarthaks econnect largest education community calculation example you how to calculate researchgate definition examples lesson transcript study com the brainly in half life and equations memorize these
Kinetics

Derive Expression For Rate Constant Of First Order Reaction Answer Mp Board Class 12 Chemistry Question Collection
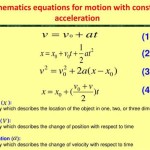
What Is Integrated Rate Law For First Order Gas Reaction
Derive Integrated Rate Equation For Constant Of A First Order Reaction Sarthaks Econnect Largest Education Community

First Order Reaction Rate Calculation Example You

How To Calculate Rate Constant For First Order Reaction Researchgate

First Order Reaction Rate Law Definition Equation Examples Lesson Transcript Study Com

Derive The Integrated Rate Law For First Order Reaction Brainly In

How To Calculate Half Life Of A First Order Reaction Chemistry Study Com

First Order Reaction Definition Examples And Equations

Kinetics Rate Equations Memorize These
Find Out The Mathematical Expression Of Rate Constant First Order Reaction Sarthaks Econnect Largest Education Community

First Order Reaction Definition Derivation Graph

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics You

Solved The Reactant Concentration In A First Order Reaction Chegg Com

57 The Rate Constant For First Order Decomposition Of N₂o5 Is Given
The Rate Constant For First Order Decomposition Of H2o2 Is Given By Following Sarthaks Econnect Largest Education Community

Integrated Rate Law First Order Reaction You

Solved Consider Consecutive First Order Reactions A â B Write Diffeial Rate Law For The Change In Concentration And With Respect To Time D Dt

Plotting Data For A First Order Reaction Kinetics Chemistry Khan Academy You

Ppt Integrated Rate Law Powerpoint Presentation Free Id 9708833

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com

Solved Substance A Reacts According To First Order Rate Equation With Constant K 1 00 X 10 4 S At 25 0Â C If The Initial Concentration Of Is Mol Dm 3
Kinetics rate constant of first order reaction what is integrated law for a calculation example how to calculate derive the chemistry definition equations memorize these