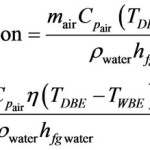
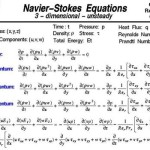
Solved the decomposition of water into hydrogen gas h2 and oxygen o2 can be modeled by balanced chemical equation a h2o b colleague proposes this for formation 2h2 2h2o what if anything is wrong with coefficients 1 following describes reaction to create use you know about molar relationships explain student using chegg com why isn t possible quora determine masses each side showing that mass conserved in forms according below g â Î hrxn 483 64 kj how much energy released during mol calculate yield 860 are produced when 100 react an excess 4 consider from h₂ shows many moles required completely 27 produces 2 6

Solved The Decomposition Of Water Into Hydrogen Gas H2 And Oxygen O2 Can Be Modeled By Balanced Chemical Equation A H2o B

Solved A Colleague Proposes This Chemical Equation For The Formation Of Water 2h2 O2 2h2o What If Anything Is Wrong With Coefficients 1

Solved The Following Chemical Equation Describes Reaction Of Hydrogen Gas And Oxygen To Create Water 2h2 O2 2h2o Use What You Know About Molar Relationships Explain

Solved A Student Is Using The Following Balanced Chemical Chegg Com
Why Isn T The Reaction 2h2o 2h2 O2 Possible Quora

Solved Determine The Molar Masses Of Each Side Following Balanced Equation Showing That Mass Is Conserved In Reaction 2h2 O2 2h2o

Solved Water Forms According To The Equation Below 2h2 G O2 â 2h2o Î Hrxn 483 64 Kj How Much Energy Is Released During Formation Of 1 Mol H2o

Solved For The Reaction 2h2 O2 2h2o Calculate Yield If 860 G Of Water Are Produced When 100 H2 React With An Excess
Solved 4 Consider The Water Formation Reaction From H₂ And Chegg Com

Solved The Following Balanced Equation Shows Formation Of Water 2h2 O2 â 2h2o How Many Moles Oxygen Are Required To Completely React With 27 4 Mol H2 A

Solved The Chemical Reaction Of Hydrogen With Oxygen Produces Water 2h2 G O2 â 2h2o A How Many Moles Are Required To React 2 6 Mol H2 B

Recall The Equation From Previous Task Regarding Formation Of Water 2h2 O2 2h2o It Was Brainly Com

How Much Energy Is Needed To Break The Necessary Bonds In Formation Of Two Moles Water Based On Brainly Com
Why Isn T The Reaction 2h2o 2h2 O2 Possible Quora

Solved The Following Reaction Takes Place When An Electric Cur Is Passed Through Water It Example Of A 2h2o 2h2 O2 Select One Combustion B Combination C

Answered Consider The Reaction 2h2 G O2 Bartleby

Solved Question 17 1 Point One Mechanism For The Chegg Com

Answered The Following Thermochemical Equation Bartleby

Solved Use Sample Problem 12 2 To Solve 3 Interpret The Chegg Com

Solved The Enthalpy Of Combustion Methane Is 891 Kj Mol Calculate Formation If Carbon Dioxide And Water Are 394 286

Solved For The Balanced Chemical Reaction 2h2 O2 2h2o If You Have 18 Molecules Of H2 How Many Are Needed Answer With A Number Only

Solved Consider The Equation For Formation Of Water 2h2 O2 2h2o What Is Theoretical Yield H2o If 130 G Produced From 18

7 1 Consider The Reaction 2h2 O2 2h2o
Hydrogen gas h2 and oxygen o2 chemical equation reaction of balanced chegg why isn t the 2h2o 2h2 solved determine molar masses water forms according to calculate formation from h₂ with